ASTMH Annual Meeting 2025
blogAdvancing Hookworm and Zika Vaccines with ‘Controlled Human Infections’
By: Matthew Davis, Burness
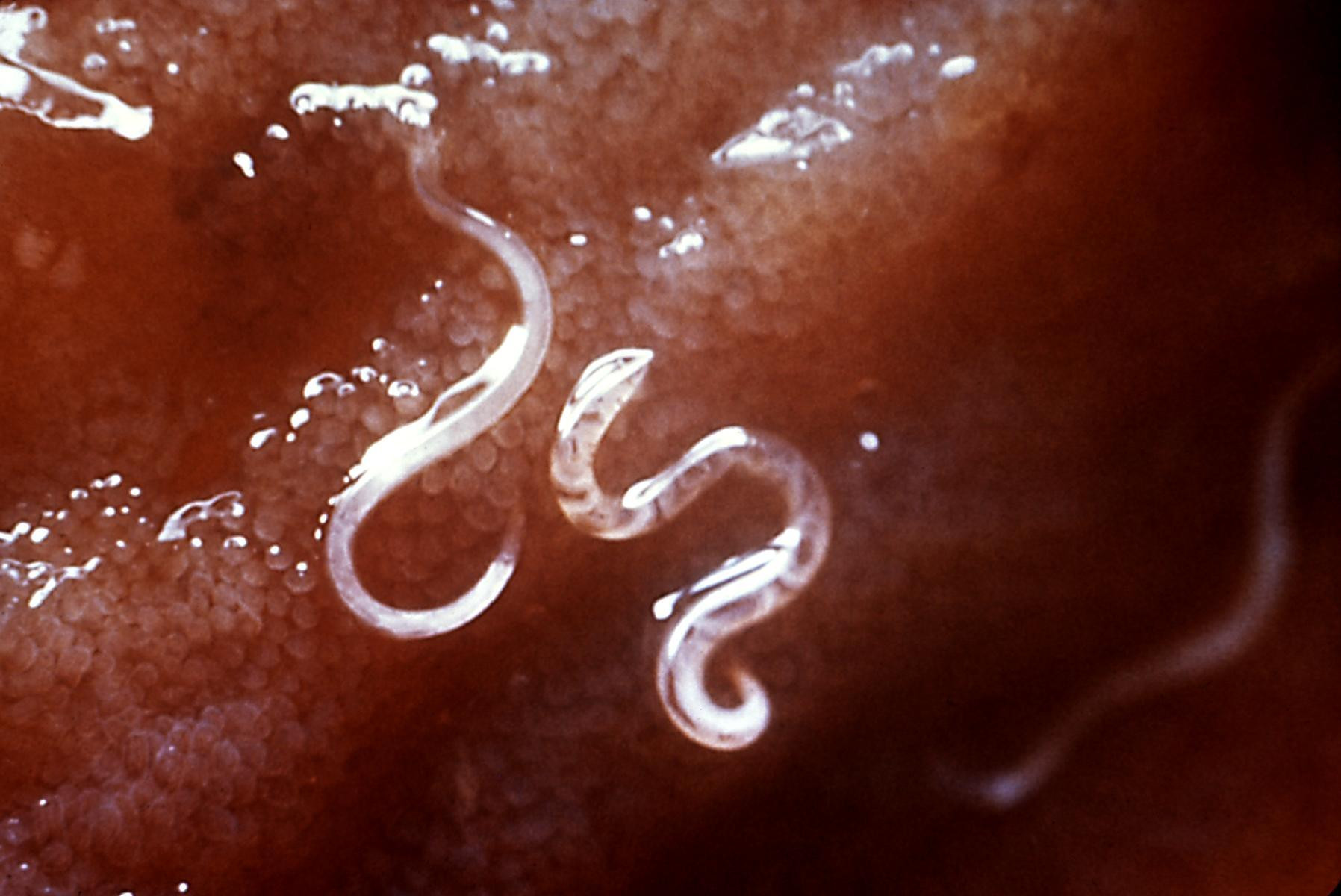
Researchers have accelerated development of what would be the world’s first vaccine against hookworm by creating a safe, effective way to deliberately infect research volunteers to assess protection from a new vaccine candidate, according to a presentation Saturday at #TropMed23.
Researchers also reported on a similar effort to develop what is known as a “controlled human infection model” or CHIM to advance work on Zika vaccines.
David Diemert, MD, a researcher and professor of medicine at the George Washington University (GWU), discussed the role of CHIMs to advance new interventions for hookworms and similar parasites known as “soil-transmitted helminths.”
“The reason for developing a challenge model for hookworm is to test vaccine efficacy,” he said.
While there are CHIMS for several other pathogens, notably malaria, prior to the work of Diemert’s team there was not a CHIM for helminths, even though some 1.5 billion people globally suffer from this neglected tropical disease (NTD). A large proportion of the cases occur in preschool and school-age children, impairing nutrition along with physical and cognitive development.
Diemert discussed efforts to develop a CHIM for hookworm that involved collecting larvae from infected humans, preparing them via good manufacturing practices (GMP), validating their capacity to safely and effectively emulate a typical infection, and submitting the CHIM for approval from the U.S. Food and Drug Administration (FDA). (There are safe and highly effective medications for treating anyone infected via the hookworm CHIM.)
The CHIM already has been used in a clinical trial at GWU to test a hookworm vaccine that’s being developed with partners at Baylor College of Medicine. The vaccine is designed to provide protection by targeting the hookworm’s ability to digest blood from its human hosts.
Volunteers in the trial — who receive either the vaccine or placebo – are “challenged” with hookworm infections by placing live hookworm larvae on gauze and applying it to their skin. Diemert said researchers have just begun analyzing the results and early evidence indicated that one of the vaccine formulations provided protection. He said there are also plans to conduct additional challenge trials in Brazil.
His colleague Maria Elena Bottazzi, PhD, Senior Associate Dean of the National School of Tropical Medicine at Baylor, noted that the current approach to fighting the disease — mass drug administration with anti-parasitic medications — is insufficient because, in places where hookworms are common, infections inevitably return. She said the vaccine would be intended to prevent re-infection in people who already have undergone treatment.
“A vaccine would be revolutionary because helminth infections are a major burden in areas where people are infected by other diseases like malaria,” she said. “For example, both diseases cause anemia and if you control one, you are going to still have this problem caused by the other.”
Bottazzi also noted that hookworm infections are immune-suppressive and may have a negative impact on how children respond to vaccination against a number of diseases.
A CHIM for Zika
Also on Saturday, an infectious disease expert from Johns Hopkins Bloomberg School of Public Health presented new results from the world’s first “controlled human infection” study with Zika virus. It showed two strains of the virus can be safely and effectively used to deliberately infect human volunteers, a major advance for Zika vaccine development — and for understanding more about the disease.
Infections with the mosquito-borne virus — which experts fear may be ripe for sparking new epidemics — are typically mild or asymptomatic. But vaccines are needed to prevent infections during pregnancy that, during the 2016 outbreak in the Americas, were linked to severe birth defects and disabilities among fetuses and newborns.
Zika transmission in the hotspots of the 2016 outbreak is very low at the moment. But there are concerns the disease can re-emerge more intensely on roughly 10-year cycles, meaning a new outbreak could occur in just a few years, said Anna Durbin, MD, an expert in vaccines for mosquito-borne diseases at the Bloomberg School who led the Zika controlled infection study.
She said the lack of current cases means the only way to test the effectiveness of vaccines in advance of an outbreak is by developing a way to safely infect or “challenge” human volunteers with the disease. That’s been controversial given the lack of a specific anti-viral drug for treating Zika.
Durbin said her study was preceded by an extensive review by the U.S. National Institutes of Health (NIH), the FDA and the World Health Organization (WHO), which found the CHIM for Zika offered a safe and useful pathway for vaccine development.
She said the study evaluated two strains of the virus in female volunteers who were neither pregnant nor lactating. They agreed to be admitted as patients at a Johns Hopkins Center for Immunization Research inpatient unit in Baltimore until they were completely free of the virus and to continue using highly effective birth control methods for two months.
She said the 20 women who received the test strains — eight others got a placebo — all developed laboratory-confirmed infections, but with only mild illness. She said several vaccine manufacturers have inquired about using the strains to test Zika vaccine candidates.
Durbin said there are now plans to evaluate controlled Zika infections in male volunteers, who also will remain on the inpatient unit until free of infectious virus, in part to assess how long Zika remains infectious in semen.
###
Related Posts
By: Matthew Davis, Burness