ASTMH Annual Meeting 2025
blogThe problem and solution to delivering safe Plasmodium vivax treatment to Cambodia’s unreached
By: Anna Erlandson, U.s. President's Malaria Initiative

Cambodia, which has the second greatest malaria burden in the Greater Mekong Subregion, aims to reach elimination for malaria-causing parasites Plasmodium falciparum and Plasmodium vivax by 2023 and 2025, respectively. The Cambodia Elimination Project (CMEP) supported by the U.S. President’s Malaria Initiative (PMI) has contributed to this intense national effort leading to remarkable progress.
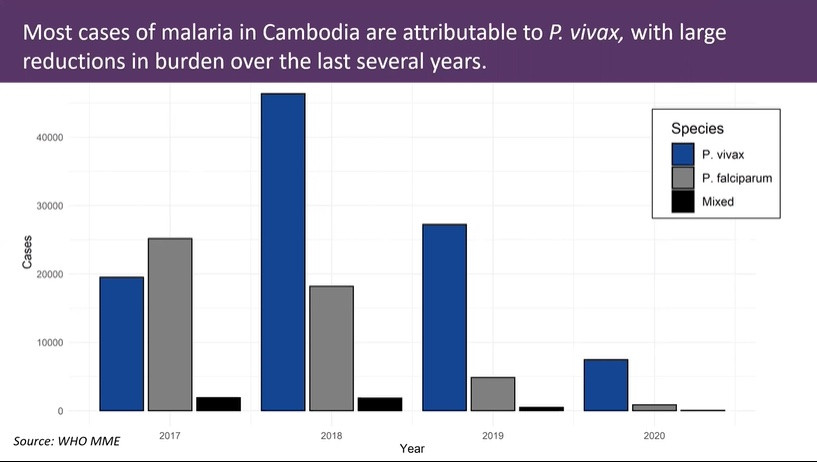
This progress, however, is under precarious threat. Cambodia’s dominant strain of malaria, P. vivax, requires an alternative drug therapy that could cause life threatening anemia in patients with glucose-6-phosphate-dehydrogenase (G6PD) deficiency, which is found in about 15% of the Cambodian population.
Differing from other parasites, P. vivax will spend weeks or months in the liver in a dormant stage called hypnozoites. Fortunately, there are alternative therapies, tafenoquine and primaquine, effective in destroying hypnozoites. Unfortunately, these drugs are also capable of destroying red blood cells in patients with G6PD deficiency. Over the past three years, P. vivax surpassed P. falciparum to become the most common type of malaria in Cambodia, and that trend is projected to continue. Therefore, in order for Cambodia to reach elimination, each patient tested for malaria and found to have P. vivax must also be tested for G6PD deficiency.
Gold standard G6PD testing requires a venous blood draw and spectrophotometry performed by trained technicians in a laboratory setting. These resources are few and far between in parts of Cambodia with high P. vivax burden, a bottleneck delaying malaria treatment which leads to repeated episodes of relapses, and ultimately delaying elimination progress.

The implementation of a rapid, point-of-care (POC) G6PD test, used alongside malaria rapid diagnostic tests, could eliminate these bottlenecks. This year, at ASTMH, Dean Sayre, on behalf of a study team consisting of PMI, CDCl, Population Services International (PSI), Pasteur Institute of Cambodia, and Cambodia’s National Center for Parasitology, Entomology, and Malaria Control, presented findings at TropMed21 from a field assessment in Cambodia on the test performance and feasibility of implementing a quantitative POC G6PD testing device. The device, STANDARD G6PD test, manufactured by SD Biosensor is similar to a glucometer used by patients with diabetes and has shown promising evidence in Thailand, the United States, Brazil and Bangladesh.

The field assessment compared the STANDARD G6PD test against gold standard spectrophotometry for G6PD testing in patients tested in the community and at health facilities. Although the results are preliminary, the STANDARD G6PD test provided a means for quantitative rapid POC G6PD testing. The POC assay was 93% (85-100%) sensitive for detecting individuals with severe G6PD deficiency with a negative predictive value of 99% (98-100%) in the community.
The results showed that the SD Biosensor provides a means for quantitative G6PD testing in the field. The sensitivity and negative predictive values nearly mirrored each other from the rapid test and spectrophotometry.
The implementation of accurate point of care malaria and G6PD testing is essential to reaching the goal of P.vivax elimination in Cambodia by 2025. By bringing care closer to people, and not waiting for people to come to care, effective diagnosis and treatment has the potential to reach the last cases of malaria in Cambodia in the hardest to reach populations.
About the author: Anna Erlandson is the communications intern at the U.S. President’s Malaria Initiative
Related Posts
By: Matthew Davis, Burness