ASTMH Annual Meeting 2025
blogFrontiers in Malaria Vaccine Development Calls for Expanding the Frontiers of Health Care Delivery
By: Daniel Hodson
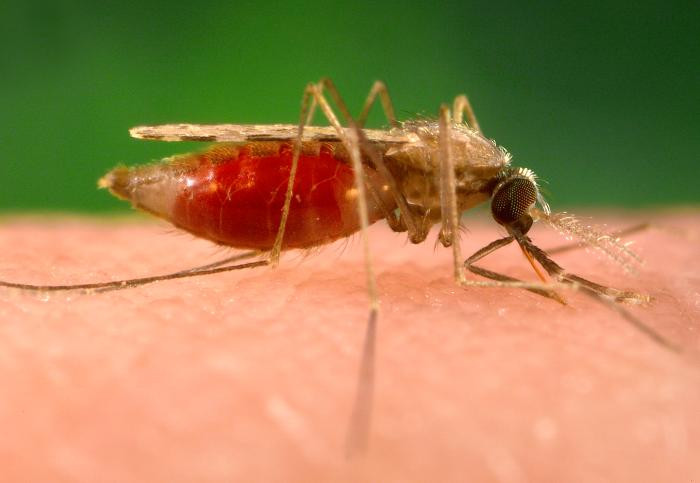
This blog was written by Daniel Z. Hodson, MS2, Yale School of Medicine, an early-career attendee to TropMed18, 2018 Kean Fellow, and guest blogger.
Working to help a rural health district in southern Senegal improve procurement and supply chain management of rapid malaria diagnostic tests and first-line antimalarials, I witnessed first-hand the burden of malaria on rural families, committees, and health structures. Despite progress made, extending access to tests and medicines to those populations living in the last mile of health care, delivery remains challenging. At the same time, I witnessed the immense success of my health district in meeting and exceeding its goals for the routine vaccinations.
In such a setting, it’s easy to see the potential of a malaria vaccine, even one offering partial protection, to have a massive impact on the burden of illness and death caused by malaria. This week at TropMed18, we heard reports from the frontiers of malaria vaccine development. Meta Roestenberg with the PfSPZ-GA1 Consortium presented Phase I data showing the safety and initial proof of efficacy for PfSPZ-GA1, the first injectable malaria vaccine assessed in humans, which contains sporozoites (the stage of the parasite that the mosquito injects into the body) genetically altered such that they will not lead to infection in humans. PfSPZ-GA1 represents an advancement over previous iterations of the PfSPZ vaccine, which uses radiation- or chemically-weakened Plasmodium falciparum sporozoites. Through analysis of existing trial data of the RTS,S malaria vaccine, Hayley Thompson of Imperial College presented results from a modeling study that showed how improving the strength with which the antibodies bind to their targets could have a greater impact on vaccine efficacy than simply increasing the amount of antibody present.
Approaching vaccine development from a different angle, Benoit Gamain of INSERM U1134 at the Université Paris Diderot Sorbonne Paris-Cité showed the safety of a placenta malaria vaccine Phase I trial, PRIMVAC, with the ultimate goal of preventing malaria from being passed through the placenta from mother to child. Earlier in the week in the Clinical Tropical Medicine II session, Jessica E. Manning with the National Institutes of Health presented Phase 1 data showing the safety of the fascinating AGS-v mosquito saliva peptide vaccine that could help prevent multiple mosquito-borne diseases by changing the way the body responds to pathogens in mosquito saliva, with the hope that the vaccine would allow the body to fight off a potential mosquito-borne infection before it took hold.
Like all interventions, a malaria vaccine will need to be implemented through existing health care delivery platforms. Improving vaccine delivery systems, expanding mass treatment and seasonal malaria chemoprophylaxis campaigns, strengthening community case detection of fever cases, and prioritizing the expansion of community-based management of fevers will all foster robust delivery platforms into which a malaria vaccine could be introduced. These presentations should remind us that investments into strengthening of these health care delivery platforms are needed to ensure that we can get malaria vaccine to the people who need it most.
Investments to build both infrastructure, such as roads and telecommunications, as well as the faith of local communities in their health systems, will allow malaria vaccines to go the last mile and reach the hardest to reach people most in need.
Related Posts
By: Matthew Davis, Burness